Once you get past your first obstacles in Another Crab’s Treasure, you can start learning new Umami abilities in your Skill Tree to be a sturdier crab than yesterday. Here are the best abilities you should prioritize leveling if you want to have a shot at finding your home.
The Best Abilities to Level Up in Another Crab’s Treasure
Abilities can be unlocked by spending Umami Crystals (the shiny purple rocks you get from defeating certain enemies or harvesting them directly) on the Moon Snail found in her cave. You learn Shelleportation by default, but all other skills are separated on Ways and must be learned manually by visiting the Snail occasionally.
There are three Ways available: the Way of the Hermit (focused on defensive maneuvers), the Way of the Predator (focused on new attacks) and the Way of the True Crab (focused on general utilities). Another Crab’s Treasure offers more than enough Umami Crystals to max them all out, but you should know which one to focus on first.
As soon as you learn Shelleportation, immediately get Streamline, Parry and Skewer to get access to all of the Ways. You can level them in any order you want, but I strongly recommend going for these first.
1 – Self Repair (Way of the True Crab)

A crab is nothing without its shell, so keep it safe at all costs! This allows you to recover the damage done to your shell after it takes a hit, giving it the potential to last forever theoretically. It won’t work like that in practice, but it’ll surely last much longer than usual. Best skill in the tree by far. And you also learn Aggravation on the way, which is a huge bonus.
2 – Spearfishing (Way of the Predator)
Give those Barbed Hooks you found some use. Grabbing certain smaller enemies may leave them immediately Capsized in front of you, ready to be immediately prayed upon. Wave Breaker is also huge but not as necessary early on.
3 – Elusive Pray (Way of the Hermit)
Your shell will eventually break, and there’s nothing you can do about it. That’s the nature of souls-like games. However, you have some breathing time with this skill. Use it to heal if necessary and wait before heading straight back into battle.
4 – Riposte (Way of the True Crab)
One of the Parry-related abilities in Another Crab’s Treasure. Makes generating Umami Charges even easier, but the timing can be weird to get used to at first. But if you enjoy delivering retribution to foes, this is one of the best ways to Capsize bosses quickly.
5 – Kintsugi (Way of the True Crab)
Despite being only one upgrade away from Self Repair, you don’t need this as much as the other skills. The extra shell can save you in a pinch, but boss arenas are usually filled with tons of spare shells you can use if needed. It’ll definitely save your life at some point but it isn’t such an urgent necessity.
Honorable Mention – Dispatch
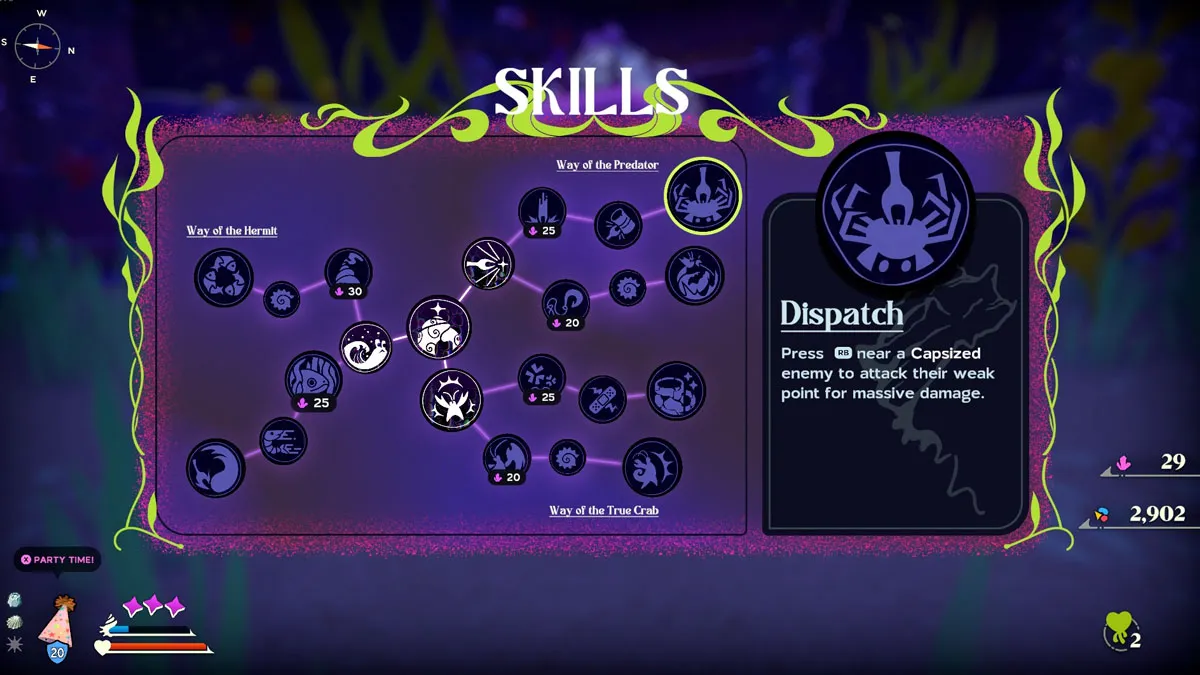
Mercilessly stabbing those annoying giant lobsters is one of the most satisfying things in the whole game. Dispatch lets you go all aggro on your opponent once they’re felled, so don’t miss the chance. It also works as a pseudo-gap-closer, so it gives you an extra edge in fights.
While you may not agree with all the skills displayed here, I highly recommend you pick at least Self Repair early, as nothing comes close to the difference that this one skill makes. Combined with a bit of extra RES early on, you’ll be an unstoppable menace against your foes.





